electron configuration of sn|Tin – Electron Configuration and Oxidation States – Sn : Tagatay Set 21, 2018 — Tin Electron Configuration. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4.
Solaire Inn & Suites features thoughtful complimentary amenities such as in room coffee makers, wireless high-speed internet access and local calls. Guests will also have access to photocopy and fax services. Explore the Foxen Canyon Wine Trail that runs through the area and boasts miles of sprawling vineyards. Golf courses, lakes and beaches .
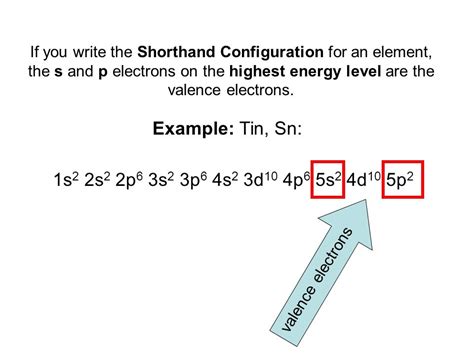
electron configuration of sn,119 rows — Mar 23, 2023 — Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full.
Click on above elements (in Periodic table) to see their information or Visit .
Hul 3, 2020 — To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the number of .

Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row .Hun 30, 2023 — Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two .
Set 21, 2018 — Tin Electron Configuration. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4.Tin electron configuration. ← Electronic configurations of elements. Sn (Tin) is an element with position number 50 in the periodic table. Located in the V period. Melting point: 232 .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram .(a) The element with electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 5; (b)A noble gases with f electrons; (c) a fifth-period element whose atoms have three unpaired p electrons; (d) First row transition metals having one 4s .Electron configuration for tin. The history of Tin. Periodic table history. Identifiers. List of unique identifiers for Tin in various chemical registry databases. Tin is a chemical .electron configuration of sn Tin – Electron Configuration and Oxidation States – SnAbr 9, 2024 — The tin electron configuration, represented as 5s2 4d10 5p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2, illustrates the precise arrangement of electrons. . Start from 1s and write till Sn for full .Ene 23, 2017 — And thus 50 electrons must be distributed according to the usual #"aufbau"# scheme...Tin lies in Group 14, and thus should have a similar electronic configuration to carbon. Can you demonstrate the similarity.
Hun 20, 2023 — The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between .Hun 27, 2024 — This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Peb 1, 2021 — An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled whenever possible. . Sn: 2: 2 6: 2 6 10: 2 6 10: 2 2 .Electronic configuration:-Electron configuration of an element shows us how the electrons are distributed in its atomic orbitals. The electron configurations of atoms follow a standard notation in which all the atomic subshells that contain electrons (with the number of electrons they hold written in superscript) are placed in a certain sequence.Hul 27, 2021 — The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .Tin occurs in 8 natural isotopes: 116 Sn, 117 Sn, 118 Sn, 119 Sn, 120 Sn, . Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The .How many protons, neutrons, and electrons are in atoms of these isotopes? Write the complete electron configuration for each isotope. Answer. Co has 27 protons, 27 electrons, and 33 neutrons: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. I has 53 protons, 53 electrons, and 78 neutrons: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5.Hun 30, 2023 — Example of Determining Energy Levels (n) For example, if we want to determine the electron configuration for Cobalt (Co) at ground state, we would first look at the row number, which is 4 according to the .La configuration électronique de Tin est 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. L'étain est l'élément chimique du tableau périodique qui appartient au groupe 14, son numéro atomique est 50 et son symbole est Sn.Reduced electronic configuration Sn: [Kr] 4d 10 5s 2 5p 2. Below is the electronic diagram of the Tin atom Distribution of electrons over energy levels in the Sn atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st level (N): 18 5-st level (O): 4. Valence electrons of Tin.
Ago 22, 2024 — This tin ion(Sn 2+) has fifty protons, sixty-nine neutrons, and forty-eight electrons. Also, tin has one more ion. That is Sn 4+. Sn – 4e – → Sn 4+ Here, the electron configuration of tin ion(Sn 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This tin ion(Sn 4+) has fifty protons, sixty-nine neutrons, and forty-six electrons.
May 20, 2018 — We expect that its electron configuration should end with s 2. Calcium’s electron configuration is [Ar]4s 2. Sn is located in the second column of the p block, so we expect that its electron configuration would end in p 2. Tin’s electron configuration is [Kr]5s 2 4d 10 5p 2.Tin – Electron Configuration and Oxidation States – SnMar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s 2 2s 2 2p 6 3s 1 configuration.
electron configuration of sn|Tin – Electron Configuration and Oxidation States – Sn
PH0 · Tin – Electron Configuration and Oxidation States – Sn
PH1 · Tin Electron Configuration (Sn) with Orbital Diagram
PH2 · Tin (Sn)
PH3 · Tin
PH4 · Electron configuration for Tin (element 50). Orbital diagram
PH5 · Electron Configuration for Sn, Sn 2+, and Sn 4+
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+)
PH8 · Chemistry of Tin (Z=50)
PH9 · 3.1: Electron Configurations
PH10 · 2.4 Electron Configurations